Bio-Nano
Understanding the world on a nano-scale
All life on Earth is built from molecules. If we want to understand biological processes and improve health and quality of life around the world, we need to look at natural phenomena at its smallest.
When it comes to our bodies, bionanotechnology is helping us to develop drug therapies, implant technology and diagnostic tools. It’s going beyond what we know, from how our cells become cancerous to how single-celled organisms adapt to their environment. Our researchers are passionate about uncovering new knowledge around some of the world’s most complex systems.
We’re all chemical beings. Chemistry is so important in our world.
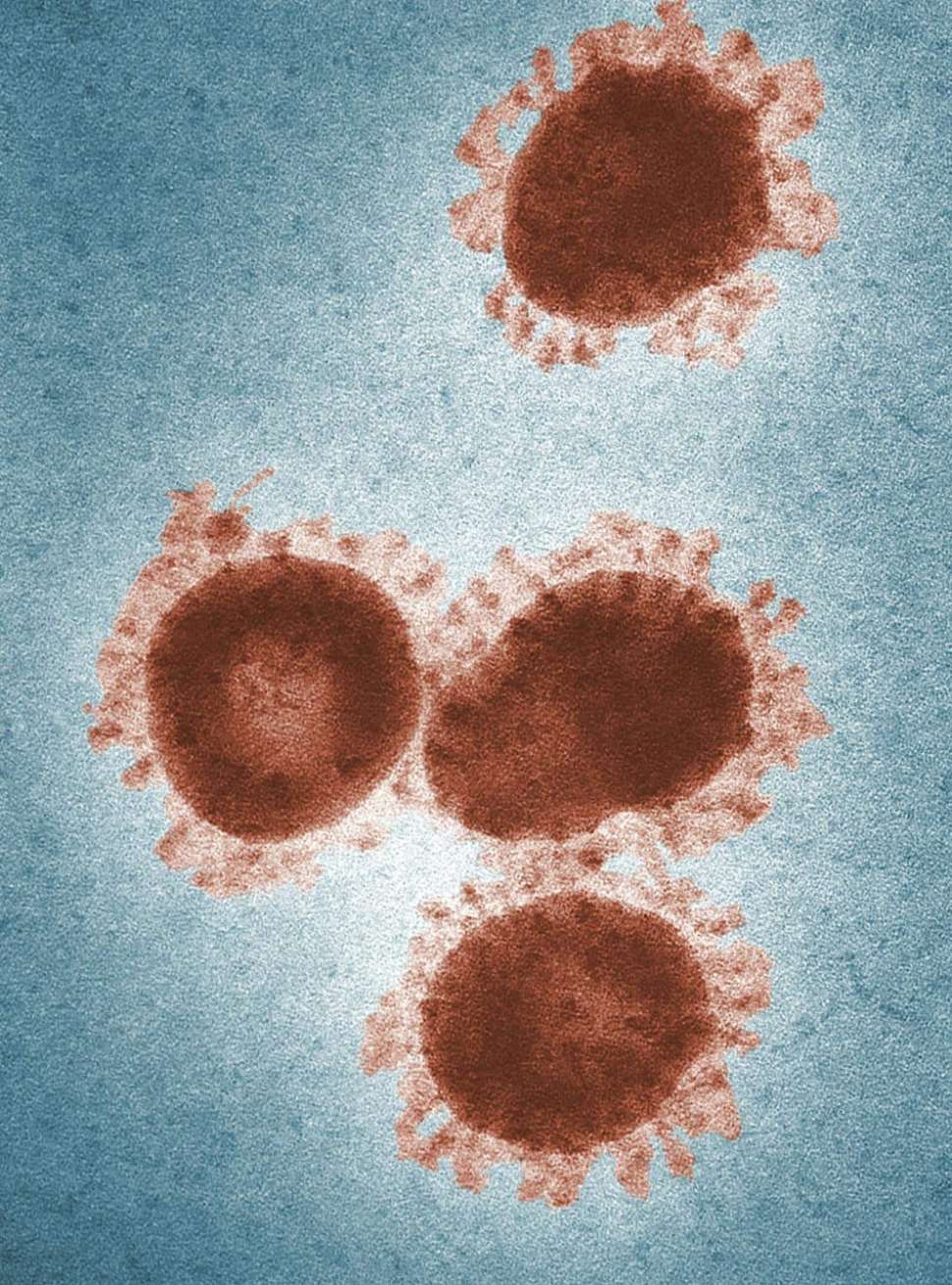
Capturing Viruses for Disease Surveillance
The Köper group are developing functionalised nanomaterials to capture viruses from the environment so that authorities can monitor the spread of infectious diseases. This research is expected to enhance our ability to detect, control, and combat, disease outbreaks in the future.
Viruses are a significant threat to human health as they can spread rapidly and cause a wide range of infectious and fatal disease. Environmental disease monitoring of pathogens such as viruses is an emerging public health approach which helps monitor the circulation of diseases in communities. This involves capturing the viruses which have been shed from infected individuals and have ended up in the local environment. The problem, however, is the tools and materials available for capturing viruses for such applications are limited or do not work very well.
Electrospun polymer nanofiber membranes have been identified as a promising material for this application. These polymer membranes feature nano and micro sized fibres with high surface area and attractive surface properties that can effectively capture viruses from environmental sources. This cross-disciplinary research within the Köper group spans surface and chemical engineering, virus composition, and biomolecule-material interactions.
My grandfather always said 'don't sleep your life away'. There are a lot of things I want to do: this research, make a difference, mentor the next generation.
Transforming how we fold proteins
A 15-hour flight from Los Angeles to Sydney can be thanked for Professor Colin Raston’s latest invention: the vortex fluidic device. This ‘eureka moment’ could transform the way we manufacture drugs.
The vortex fluidic device combines water, a liquid solvent and a laser in a rapidly spinning glass tube; it has been described like a small, tubular laundry washing machine with lasers. The device has the ability to untangle and slice carbon nanotubes that are just a few nanometres in diameter (i.e. tiny—about the width of a small virus) with unprecedented precision. Because carbon nanotubes are 200 times stronger and five times more flexible than steel, as well as five times more effective in conducting electricity than copper wires, this discovery could have wide ranging impact.
Carbon nanotubes are already being used for highly targeted drug delivery in cancer therapy, but scientists have always struggled to properly harness the material. The vortex fluidic device not only makes the process more effective and maintains the properties of the carbon nanotubes, but also offers a cleaner, cheaper and faster solution. Using the vortex fluidic device the common cancer treatment drug, carboplatin, used against ovarian and lung cancers, can be made four-and-a-half times more potent. The device could also enable small scale drug production in remote areas or disaster zones.

I like to work with people in engineering, bioimaging, medicine, chemistry, mechanical engineering, biomechanical engineering... The best ideas come from working with people with different perspectives.
Preferring to pee at home
Urine tests are a staple diagnostic tool for doctors. It makes sense that Associate Professor Youhong Tang would want to make them more flexible, responsive and easy to administer.
Youhong works with medical students and collaborators to examine the biomarkers in urine and develop a way for people to quickly and easily test their urine at home. Imagine using a slip of paper—like testing the pH of a home pool—and an app to send through the results to your doctor. For people living in remote areas or who need regular tests, this level of convenience could be a huge relief.
But for Youhong, the most exciting part is collaborating with people in different fields and with different perspectives. A multidisciplinary group leads to new ideas, new challenges, and new solutions.
Battling antimicrobial resistance
Developing novel antibacterial strategies has become increasingly urgent. Modern healthcare is under threat due to the increasing prevalence of antimicrobial resistant bacteria and persistent biofilms. Aggregation-induced emission-based photosensitizers are promising candidates due to their unique photodynamic and photothermal properties.
Professor Youhong Tang, and collaborators are applying a novel antibacterial coating that integrates plasma polymerization with aggregation-induced emission photosensitizers to selectively eradicate pathogenic bacteria through light-triggered reactive oxygen species generation, presenting a promising approach to combat antibiotic resistance.
The building blocks of science
Professor Jim Mitchell has a different approach to his colleagues. He’s not interested in creating or how his research could be applied. He leaves that part to his students—and he’s good at it, once awarded the best PhD supervisor in Australia.
Jim is studying diatoms: the most successful single-celled photosynthetic organisms in the world. Jim first discovered diatoms in 1978 when he was in Antarctica. They’re made of opal glass, they range from five micrometres to a couple of millimetres, and they exist in the most amazing variety of shapes—for which there is no explanation. There are probably over 100,000, or maybe 200,000, species. Each species has adapted to its surroundings. Diatoms dominate the ocean and rivers. They collect food by dissolving molecules using tiny holes (around a micrometre, which is about a 1000th of a millimetre).
Understanding why they’re so successful, how they work, and what tricks they use will form the building blocks of science. It might take 10 years, 30 years, more than his lifetime. In 1959, scientist Richard Feynman gave a presentation entitled, 'There’s more room at the bottom.’ Meaning, there’s so much we don’t know about small scales. We still need to apply Newton’s principles of physics. It’s not the type of thing funded by government or industry, who want quick returns. But if we don’t find those building blocks, we’ll eventually run out of new science. Jim isn’t worried if his work doesn’t make an impact in five years or 50. He’s going for long term impact—for profound understandings of biology that will drive science forward.

It may take 10 or even 50 years for my research to make an impact. It's a slow process, but I'm fairly patient.
Detecting cancer earlier
What if we could see cells inside the body without cutting people open? Dr Justin Chalker and his team are working to detect protein cancer cells earlier than normal. They’re using chemistry to add a radioactive tag to protein. As the protein moves around the body, it can be tracked with imaging.
Scientists can learn more about biological functions, and doctors can diagnose illness and disease. In the future, these well-defined protein tools could be used to control the release of treatments. This means that radiation for cancer treatments can be given in a controlled manner.
I'm interested in applying physics to chemistry in order to understand biological processes. I call myself a bio-physical chemist.
Building a cell membrane
Professor Ingo Köper and his team have built model structures that look and act like cell membranes. Why? Cell membranes are super complicated structures that have their own reactions to different substances. We don’t yet have a way to easily examine and test interactions at cell interfaces. The artificial membrane Ingo has created can last several months, while natural biological membranes might last a week. It’s pure science that they’re doing—trying to understand membrane interaction phenomena.
The models can be used to investigate how nanoparticles or drugs interact with membranes. Ingo imagines it could contribute to targeted drug delivery. If you take a Panadol for a headache, it goes through your stomach and intestines and eventually into your blood, and then through your whole body to eventually reach somewhere in your brain. Of the 500mg you take, only a small fraction reaches the point of pain. Apply the same idea to cancer, and that’s why you get a range of side effects—the drugs attack your whole body. Ingo’s research could help build drugs that might still travel the whole body but will only release the drug once they sense a cancer cell. Or again, the membranes can be made to look like bacterial membranes, allowing us to test how antibiotics work and help to discover new alternatives.

![]()
Sturt Rd, Bedford Park
South Australia 5042
South Australia | Northern Territory
Global | Online
CRICOS Provider: 00114A TEQSA Provider ID: PRV12097 TEQSA category: Australian University








